Retinal and Macular Degeneration Clinical Research Group
The Retinal and Macular Degeneration Clinical Research Group focuses on translational and clinical research in retinal disease. Its goal is to generate the evidence base required for the development and clinical implementation of novel therapeutic strategies.
Key projects and focus areas
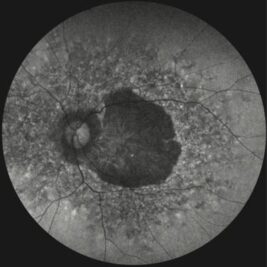
A key milestone is the EyeConic Study, a global multicenter ocular imaging initiative conducted in collaboration with more than 15 international clinical sites. EyeConic systematically characterises residual retinal structure in patients with inherited retinal degenerations (IRDs). Using AI-based image analysis, EyeConic demonstrated that a significant proportion of patients with generalised IRDs retain preserved foveal tissue, even in advanced disease stages. This finding closes a major knowledge gap and provides strong evidence supporting the feasibility of photoreceptor-targeted vision restoration therapies.
Disease-specific:
- EyeConic-Pediatric: Assessing foveal tissue preservation in blind pediatric patients with IRD.
- EyeConic-GA: Investigating residual retinal structure in Geographic Atrophy (GA) due to age-related macular degeneration to evaluate the potential for vision restoration therapies.
- Stargardt disease: Focusing on early-stage Stargardt disease, mutation-specific progression, and biomarkers for clinical trial stratification. We envision enrolling patients into the first-in-human clinical trial assessing base-editing-mediated gene correction.
The EyeConic framework has established a robust methodological basis for large-scale multicenter imaging research, ensuring that quantitative data are reproducible and comparable across devices, sites, and patient populations.
Beyond EyeConic, our broader vision is to establish an integrated imaging and clinical research framework that connects multimodal imaging and advanced AI-based image analysis with clinical, functional, and genetic datasets. We aim to accelerate the development of new therapeutic options for patients and reduce the risk of clinical trial failure arising from patient-specific factors. Our work includes identifying biomarkers, creating disease atlases and providing predictive models of disease progression.
Our group operates within an international collaborative network, partnering with leading institutions across Europe, North America, Asia and leveraging IOB’s interdisciplinary environment to connect fundamental discovery with clinical translation.
We welcome new clinical collaborators, researchers and fellows interested in retinal imaging, AI-driven analysis, or vision restoration therapies.
For more information or to explore collaboration opportunities, contact us at: clinical.retina@iob.ch
Further links
Clinical Trial Registrations
EyeConic: Qualification for Cone-Optogenetics, NCT05294978 (EyeConic)